
Alloys developed for High Temperature(Superalloys) Applications require combinations of mechanical strength, microstructural stability and corrosion/oxidation resistance. Nickel base superalloys have been traditionally the prime materials utilized for hot section components of aircraft turbine engines. Nevertheless, due to their limited melting temperatures, alloys based on intermetallic compounds, such as TiAl base alloys, have emerged as high temperature materials and intensively developed with the main aim to replace nickel based superalloys. For applications in steam power plants operated at lower temperatures, ferritic high temperature alloys still attract high attention, and therefore, development of these alloys is in progress. This paper highlights the important metallurgical parameters of high temperature alloys and describes few efforts in the development of Fe-Ni-Al based alloys containing B2-(Fe,Ni)Al precipitates, oxide dispersion strengthening (ODS) ferritic steels and titanium aluminide based alloys include important protection system of aluminide coatings.
INTRODUCTION
High temperature operations, such as those found in aircraft and land based turbine engines as well as boilers, require materials which have a good combination of mechanical properties, microstructural stability and corrosion resistance at relatively high temperatures. Especially for air craft turbine blades, these components are subjected to a number of extreme external conditions, such as high tensile loads and corrosive environments, all of which occur under thermal cycling conditions due to the relatively short cooling-heating cycle of the engines. Similarly, components in coal-fired power plants, such as heat exchangers, are exposed at high temperatures in environments containing corrosive species and particulates. Especially for fossil fuel engines operating at higher efficiency is demanded due to the depletion of global fossil fuel resources as well as requirement for reducing global warming. Thermodynamic principle has clearly suggested the requirement of higher operating temperatures for higher efficiency of the engines. Obviously, this will give further challenge to continuously improve high temperature alloys before brittle non-metallic materials, such as oxide systems, can be toughened.
The target for higher efficiency in coal-fired advanced ultra-super critical (A-USC) steam cycle needs advanced materials that capable to be operated at high temperatures (≥700℃) and pressures (≥350 bar) 1 . Advanced steels, stainless steels as well as nickel based alloys are considered as the materials that can fulfill this requirement. Turbine blades of aircraft engines are the parts that experience extreme operating conditions, both at high pressure (>40 bar) and temperatures (>1600℃) 2 . Materials used for these applications have been studied and developed rigorously, and the results may be shared for the application of high temperature alloys for land base turbine engines as well as steam power plant.
STRENGTHENING MECHANISMS OF HIGH TEMPERATURE ALLOYS
The strength of most alloys are normally decreases with temperatures, except some intermetallic compounds, such as γ’-Ni3Al which increases with temperatures up to about 900o C 4 . At any temperature, pure metals are usually weak and easily attacked by corrosive environment. Therefore, metals are alloyed with other elements in order to have strengthening as well as corrosion resistance. For corrosion protection, the alloying elements, such as Cr, Al or Si, should be dissolved homogenously in the matrix of base metals, either Fe, Ni, Co or Fe-Ni so they have readiness to form protective scales of Al2O3, Cr2O3, SiO2 on the surface of the alloys. In addition, the dissolved elements can provide strengthening to the alloys through solid solution strengthening. When addition of the alloying elements higher than their solid solubility limit, second or third phase precipitates can occur and these provide another mode of strengthening mechanism, i.e., precipitation strengthening or hardening. Precipitates of γ’-Ni3(Al,Ti) has been well recognized as the main strengthener for nickel-based superalloys. The addition of carbon in Fe, Ni and Co based-alloys provide various types of carbides precipitates, such as M23C6, MC, M6C, M7C3, where M occupied by refractory elements (Mo, W, V, Nb) or Cr, and these contribute to the additional precipitation strengthening for the alloys. When these carbides precipitate along grain boundaries, they increase the creep resistance of polycrystalline alloys. However, because the precipitates are metastable, they will coarsen when heated at high temperatures, so that the strength and creep resistance of the alloy decreases with time. Efforts have been done to involve more stable nano size oxide particles such as Y2O3, ThO2 and ZrO2 that can disperse homogenously in the alloy grains through mechanical alloying process 5 . This oxide dispersion strengthening (ODS) alloys have higher stability since the oxide particles will not coarsen. Other strengthening mechanisms that apply for room temperature applications, such as strain hardening and martensitic strengthening are not effective for high temperature applications due to recrystallization and grain growth phenomena.
ALLOY DEGRADATION AT HIGH TEMPERATURES
Movement of atoms or ions in the materials increases at high temperatures due to the involvement of activation energy. In addition, the concentration of vacancy that responsible for diffusion of atoms and ions increase exponentially with temperatures. Therefore, any changing that occur in alloys, both in the interior as well as on the surface of the materials that reduce their properties, increases significantly with temperatures. High temperature alloys are normally designed to reduce this changing. Oxidation, hot corrosion, creep, thermal fatigue and microstructural changing are modes of alloy degradation occur at high temperatures. Oxidation and hot corrosion are considered as the worst alloy degradation modes because of their close relation with environment that relatively difficult to control. Combination of high temperature operation and contaminants in environment, such as sulfur, sodium, halides and vanadium, resulted in the formation of a dangerous high temperature corrosion mode named hot corrosion. Unlike high temperature oxidation that produce relatively uniform and highly predictable scales as corrosion products, this type of corrosion has pitting type with rapid rate and often unpredictable. Therefore, catastrophic failure of the components may occur due to this hot corrosion since the load-carrying ability of the material is reduced rapidly. Extensive studies have been conducted to investigate the phenomena of hot corrosion, especially in air-craft turbine engines 6,7,8. Hot corrosion may be defined as accelerated attack of molten sodium salts and oxides mixture such as NaCl, Na2SO4 and V2O5 deposited from environment contaminants at high temperatures. Two forms of hot corrosion attack have been identified 9 , i.e., Type 1 hot corrosion, which is hot corrosion occur at high temperatures (850-950o C) or usually named as high temperature hot corrosion (HTHC), and Type 2 hot corrosion, which is hot corrosion that occur at relatively low temperatures (650-850o C) or normally called as low temperature hot corrosion (LTHC). Chemical composition of the alloys or coatings and environment contaminants as well as the operating temperatures will define the hot corrosion type that applies. In HTHC the dominant deposit is fused alkali salt, especially Na2SO4. This deposit formed as the results of reactions between SO2 from the fuel combustion, NaCl from fuel or environment that close to coastal and H2O as shown in Eq. (1) 6 :
2NaCl + SO2 + H2O + 1/2O2 → Na2SO4 + 2HCl
At 850-950o C, the deposit will be in liquid phase so that it can cover the whole surface of the scales of the components. Na2SO4 that melts at about 880o C firstly react with the oxides in the protective scale, such as Cr2O3 or salt fluxing 9 and this leads to further oxidation of Cr that occasionally makes depletion of Cr in substrate underneath the scale. Finally, oxidation will occur with the base metals, Fe or Ni, and produce porous scales. This oxidation will release elemental sulfur, and sulfides of base metals with low melting point will form. This causes peeling of the surface metals and the corrosion product is characterized by base metal oxide precipitates dispersed in a film of salt mixtures. Other fuel or atmosphere contaminants, such as vanadium and phosphorous, might mix together with Na2SO4 to produce lower melting point salt mixtures, so that increasing the degree of attack. Excess of NaCl can produce eutectic of NaCl and Na2SO4 that melt at 620o C. The LTHC occur at lower temperatures and characterized by pitting type attack. This corrosion is mainly due to the formation of low melting point eutectics of the mixture between Na2SO4 and base metal sulfates, such as NiSO4 and FeSO4. The eutectics have melting points of about 550o C. NiSO4 and FeSO4 formed as the result of the reaction between base metal oxides and SO3 in the combustion gas. High partial pressure of SO3 is required for the LTHC to proceed.
PROTECTION OF THE ALLOYS
In order for scales can act as protective layer on alloy surface, they should consist of oxides that thermodynamically stable at high temperature, have high melting point, low vapor pressure, slow formation rate, high adherence on the alloy surface, low thermal expansion, and good erosion resistance. At temperatures higher than 560o C, Fe in iron based alloys oxidized to form FeO. However, this oxide has high oxidation rate, therefore FeO is a non-protective scale, and similarly NiO in Ni-based alloys and TiO2 in titanium based alloys. Almost all high temperature alloys utilized protective scales of Al2O3, Cr2O3 or SiO2. Among these three protective scales, Al2O3 has higher stability at temperatures higher than 1000o C, since Cr2O3 or SiO2 can form high volatile oxides, such as CrO3, However, Cr2O3 has better protective scale for hot corrosion mode. The primary reason for the addition of aluminium in Fe and Ni based alloys is to provide alloy strength through formation of coherent intermetallic phase precipitates of γ’-Ni3(Al). Moreover, the maximum chemical content of aluminium necessary to optimize the strength in these alloys is considered too low to produce the protective scale of Al2O3 10. Furthermore, it has been reported 11 that increasing the aluminium content in nickel-based superalloys reduces the solubility of chromium in the matrix and therefore decreases the hot corrosion resistance of the material. Therefore, to provide superalloys with high strength and good oxidation and corrosion resistance, coatings based on the nickel aluminide E-NiAl are applied on the surface of the materials
Fe AND Ni BASED ALLOYS FOR HIGH TEMPERATURE APPLICATIONS
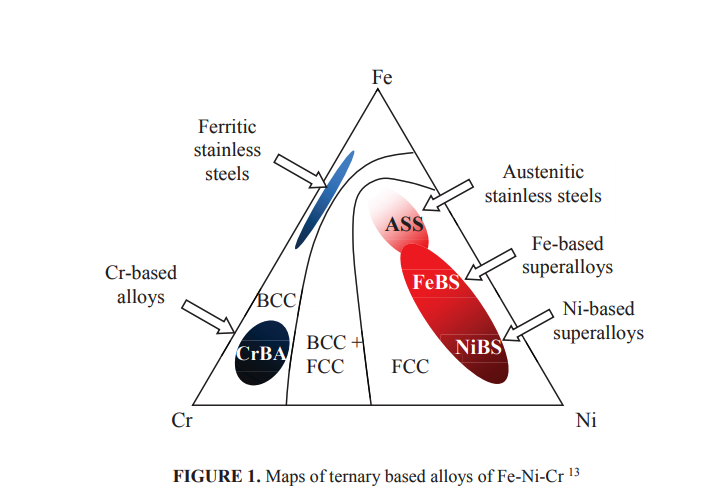
Iron based alloys have been developed for high temperature applications, such as tubes of steam power plant, from very simple Fe-2.25Cr-1Mo steels that only provide about 42% efficiency up to advanced Fe-12Cr based steels containing refractory elements of W, Nb and V that provide carbide particles strengthening and give efficiency of the power plant at about 50% 12. Higher efficiency might be obtained from nickel based alloys. The Ni-Cr-Al systems are the basic for development of nickel-based superalloys. Other alloying elements such as Ti and Mo may be involved for precipitation of intermetallic compounds, especially γ’-Ni3(Al,Ti) and carbide precipitates of MC, M23C6, M7C3 or M6C4 . However, Fe-Ni based alloys with α-ferrite or γ-austenite matrix still attract high attention for high temperature applications since this group of alloys are less expensive compared with Ni-based alloys. Schematic ternary phase diagram of Ni-Fe-Cr system, as shown in Fig. 1, can be used as the basic for development of both iron and nickel based alloys. Various alloy systems may be developed from alloys of rich in Ni up to those rich in Fe, all of which may contain minimum Cr of about 12% for Cr2O3 protective scale formation can occur all over the surface of the alloys.
Development of B2-(Fe,Ni)Al in α and γ Matrix
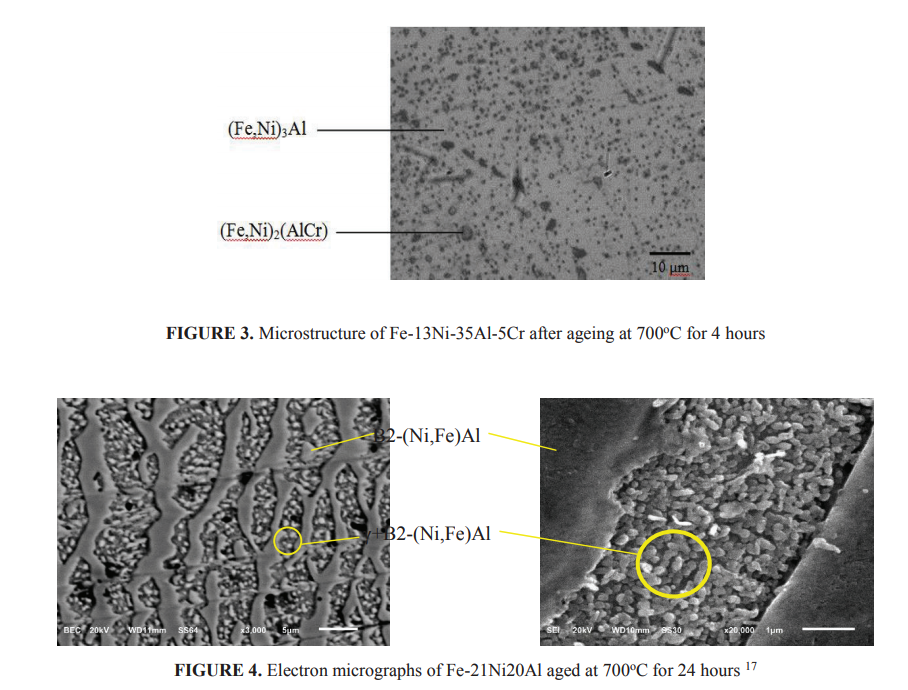
The replacement of some Cr with Al as alloying elements are of great interest especially for development of FeNi-Al based alloys 14 as these materials are relatively cheaper, lighter and stronger as a result of the dispersion of intermetallic compound precipitates of B2-(Fe,Ni)Al 15,16. Investigation on the effect of aluminum addition on the microstructural evolution of Fe-Ni-Al alloys at high temperatures has been recently conducted 17. Previously Fahmi 18 has studied the Fe-Ni-Al-Cr i.e., Fe-13.7Ni-35Al-2Cr, Fe-13Ni-35Al-5Cr and Fe-12Ni-35Al-10Cr (all composition are in weight percent unless stated otherwise). The materials were made from low carbon FeNi shots containing 76.7 wt.%Fe, 22.7 wt.%Ni, 0.3 wt.%Co, 0.1 wt%Cr, 0.1 wt%Si, 0.01 wt.%C, 0.01 wt.%P and 0.017 wt.%S produced by PT. Antam Tbk. and pure aluminium ingot obtained from PT. Inalum Indonesia. Melting in a single electrode DC arc furnace purged with high purity argon was carried out for preparing the alloys. The as-cast alloys were homogenized at 1100℃ in an electrical tube furnace in an argon atmosphere. Figure 2 shows the buttons of as homogenized alloy samples. Fahmi 18 obtained two important intermetallic compounds of (Fe,Ni)3Al as the matrix and (Fe,Ni)2(Al,Cr) as precipitate (Fig. 3). Since these materials are brittle, Fitriani 17 selected chemical composition of Fe-21Ni-14Al, Fe21Ni-17Al and Fe-21Ni-20Al in order to obtained high concentration of B2-(Fe,Ni)Al in the matrix of α-Fe and γ-Fe. After solution treated at 1100o C, all samples were aged at 600, 700 and 800o C for 6, 12, 24 and 36 hours. All alloys contain B2-(Fe,Ni)Al phase, one of which is shown in Fig. 4. This study revealed that addition of Al into FeNi promote transition of the intermetallic precipitates, varied from NiAl, FeAl, and (Ni,Fe)Al. The matrix of γ-Fe was found in the alloy of highest Al content, i.e., Fe-21Ni-20Al. However, as seen in Fig. 4, large size B2-(Fe,Ni)Al occurred.
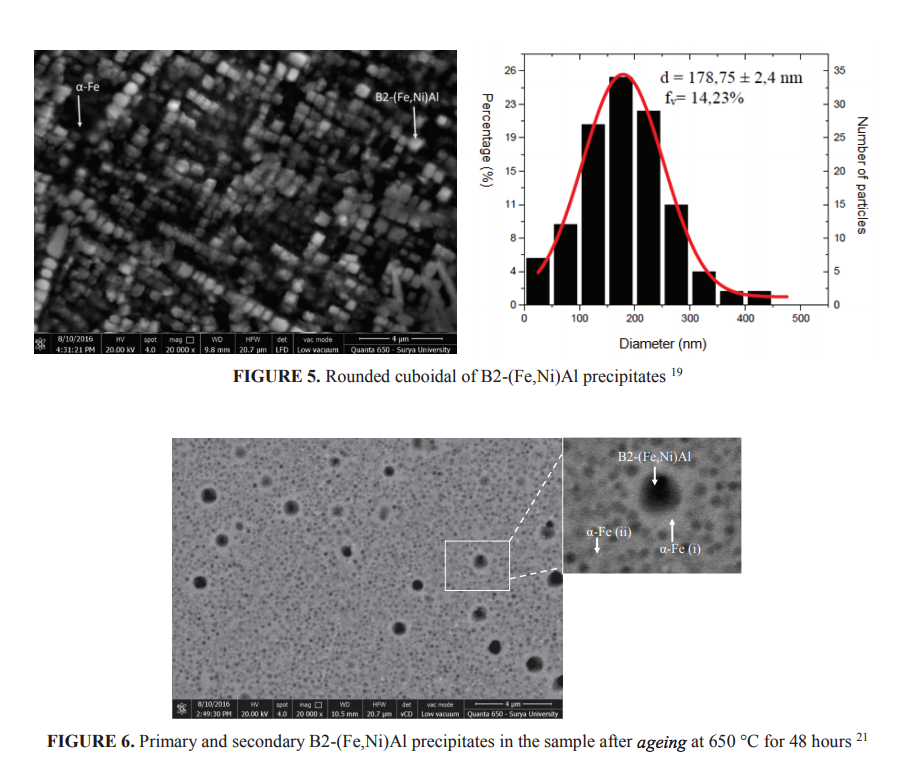
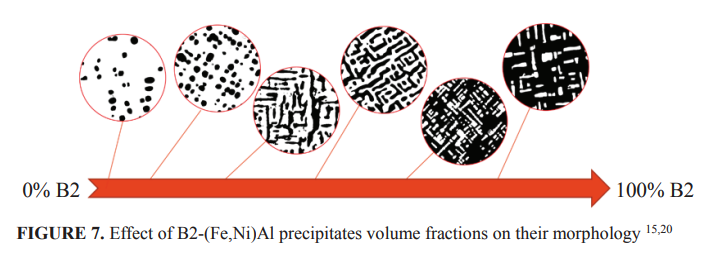
To avoid large size of B2-(Fe,Ni)Al phase and to obtain α-Fe matrix, Fantowi 19, Adli 20 and Yanas 21 investigated the development of Fe-Ni-Al-Cr based alloys of Fe-16Ni-9Al with different Cr content of 2.5%, 5% and 7.5%. To develop very fine precipitates, the alloys were solution treated at 1100o C and aged for various times at 650, 750 and 850o C. Due to the limited space available, only few results were presented in this paper. Figure 5 shows nano size rounded cuboidal B2-(Fe,Ni)Al precipitates distributed homogenously in the matrix of α-Fe. The alloy had chemical composition of Fe-16Ni-9Al-5Cr. The particle size, however, varied from very small size up to about 500 nm, with the average of roughly 180 nm. The precipitates have crystallographic orientation of<100>since the ferrite matrix has body centered cubic. The study also showed that the morphology and the volume fraction of the B2-(Fe,Ni)Al phase varies with the chromium content in the alloys. Since Cr is a ferrite stabilizer, increasing Cr content in the alloys reduces the B2-(Fe,Ni)Al volume fractions. The volume fraction of the B2-(Fe,Ni)Al reduces in alloy containing higher Cr, such those find in Fe-16Ni-9Al-7.5Cr. Moreover, the secondary B2-(Fe,Ni)Al precipitates coarsened during ageing resulted in the consumption of surrounding smaller B2-(Fe,Ni)Al precipitates as shown in Fig. 6. Similar with that Stallybrass has previously informed, this study also found that rounded - cuboid occur when the volume fraction less than about 20% and tend to be elongated when the volume fraction increases, as schematically shown in Fig. 7.
Development of ODS Ferritic Steels
Coarsening of B2-(Fe,Ni)Al precipitates in ferrite matrix as well as other precipitates, such as carbides and J'- Ni3(Al,Ti) have been found 21,3. This can occur at high temperatures due to the tendency of alloys to reduce their free energy. Consequently, the strength of the alloys decreases with time of operation since the distance between particles widen with time. However, for oxide particles such as Y2O3, ThO2 or ZrO2 dispersed homogeneously in the alloy matrixs, due to their extremely low solubility in the matrix, they would not coarsen at high temperatures. Therefore, these systems have been considered very effective for high temperature strengthening.
Alloys developed for high temperature applications














